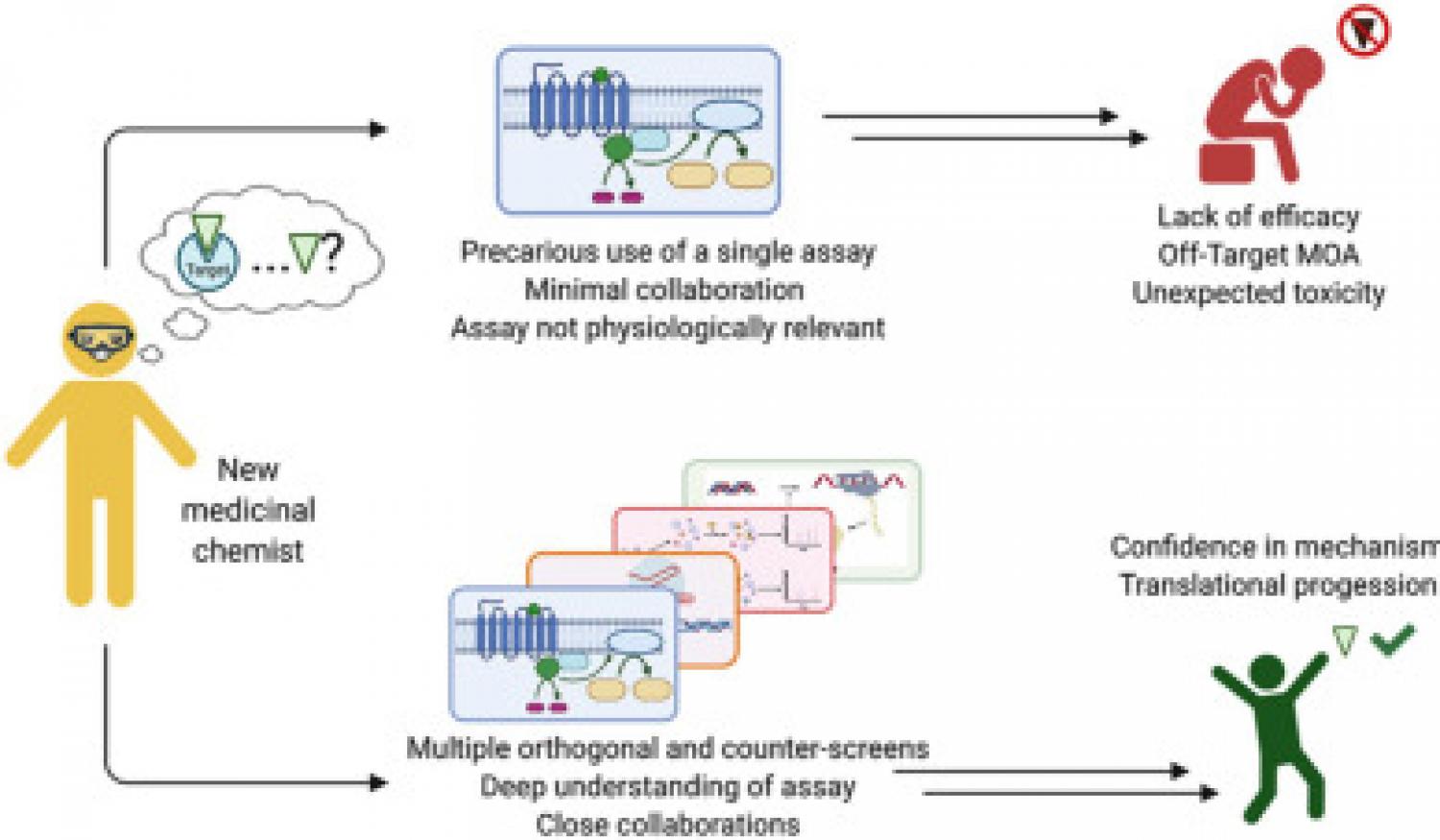
A diverse range of biochemical and cellular assays are used by medicinal chemists to guide compound optimization. The data collected from these assays influence decisions taken on structure-activity relationship (SAR) campaigns. Therefore, it is paramount that medicinal chemists have a solid understanding of the strengths and limitations of each assay being used to characterize synthesized analogs. For the successful execution of a medicinal chemistry campaign, it is our contention that an early partnership among assay biologists, informaticians, and medicinal chemists must exist. Their combined skill sets are necessary to not only design and develop robust assays but also implement an effective screening cascade in which multiple orthogonal and counter assays are selected to validate the activity and target(s) of the synthesized compounds. We review multiple cases of drug and chemical probe discovery from collaborative National Center for Advancing Translational Sciences/National Institutes of Health projects and published scientific literature in which the evaluation of compounds in secondary or orthogonal assays led to the discovery of unexpected activities, forcing a reconsideration of the original assay design that was used to discover the biological activity of the compound. Using these retrospective case studies, the goal of this Perspective is to hedge toward the development of physiologically relevant assays that are able to capture the true bioactivity of compounds being developed in a medicinal chemistry campaign.
