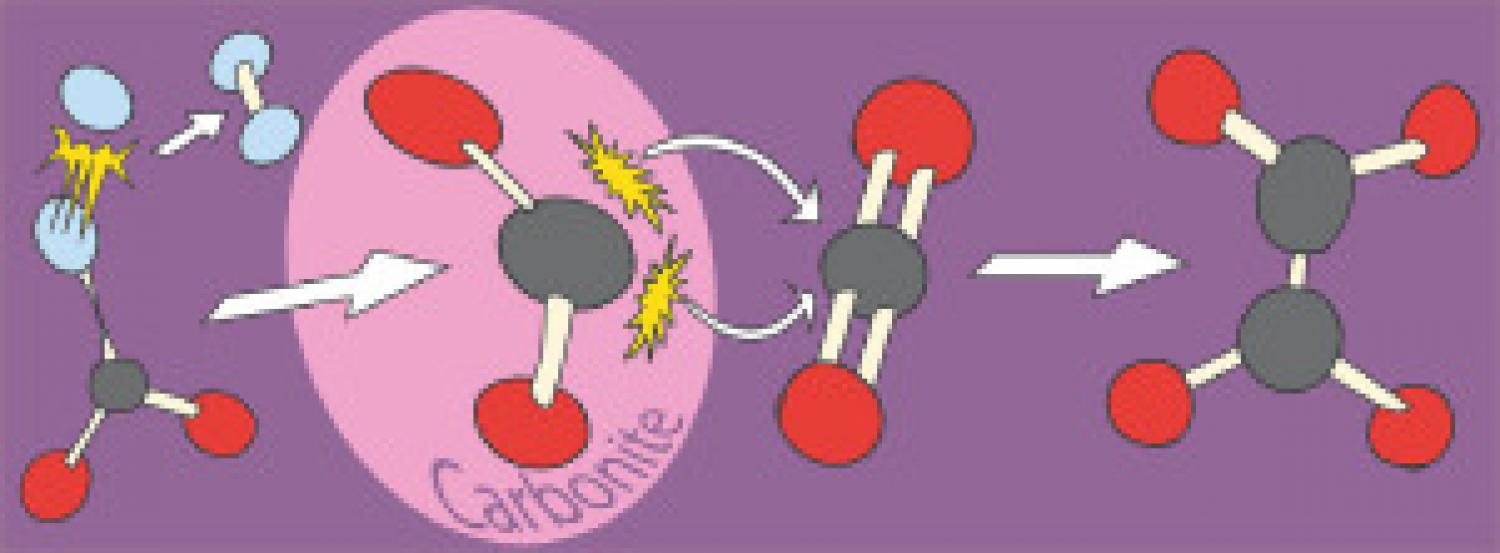
Conversion of CO2 to valuable chemicals such as polymers via the electrochemical reduction of CO2 to formate followed by the formate to oxalate coupling reaction (FOCR) is an interesting concept to replace fossil feedstocks with renewable ones. Yet, the activation of CO2 is challenging and energy-intensive and today the production of one oxalate molecule first requires the reduction of two CO2 molecules. Recently we confirmed the crucial role of the reactive carbonite intermediate in the FOCR. Due to its high reactivity, this intermediate might also be a strong enough nucleophile to react with CO2 directly. If this is the case, we can form oxalate directly from CO2 and formate and avoid the need for double electrochemical CO2 reduction in oxalate production. In this work, we successfully established the conversion of CO2 (with a theoretical yield of 52%) to oxalate (via the reaction with carbonite), as well as to formate and carbonate. The direct reaction of the reactive carbonite intermediate with CO2 was the dominant pathway for CO2 incorporation in oxalate. For enhancing the CO2 incorporation in oxalate, we found a reaction temperature of 200°C, stoichiometric amounts of the base, and the presence of CO2 in the supercritical state most suitable. The residence time is strongly depending on the reactor type but should be kept to a minimum to avoid carbonate formation. The presence of high amounts of hydride and supercritical CO2 appeared to also cause the formation of carbonates as a side-product. The carbonate formation increased with higher temperatures and longer reaction times, which suggests a consecutive decomposition of oxalate formed in the reaction.
