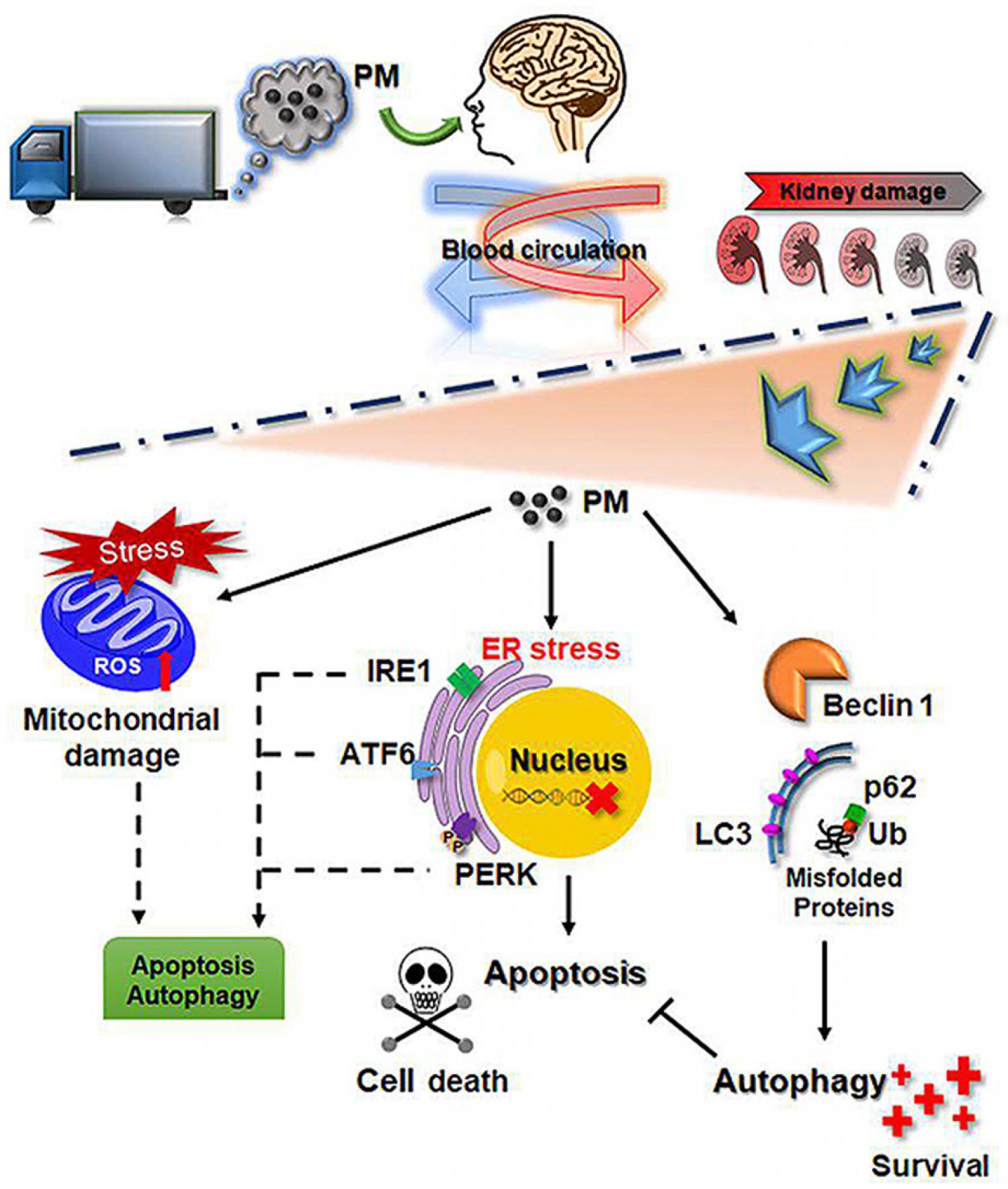
Traffic emission is responsible for most small-sized particulate matter (PM) air pollution in urban areas. Several recent studies have indicated that traffic-related PM may aggravate kidney disease. Furthermore, exposure to particulate air pollution may be related to the risk of chronic kidney disease (CKD). However, the underlying molecular mechanisms have not been adequately addressed. In the present study, we studied the mechanisms of renal damage that might be associated with exposure to PM. In a real world of whole-body exposure to traffic-related PM model for 3–6 months, PM in urban ambient air can affect kidney function and induce autophagy, endoplasmic reticulum (ER) stress and apoptosis in kidney tissues. Exposure to traffic-related diesel particulate matter (DPM) led to a reduction in cell viability in human kidney tubular epithelial cells HK-2. DPM increased mitochondrial reactive oxygen species (ROS) and decreased the mitochondrial membrane potential. Furthermore, DPM induced ER stress and activated the unfolded protein response (UPR) pathway. Eventually, DPM exposure induced caspase pathways and triggered apoptosis. In addition, DPM induced autophagy through the inhibition of the Akt/mTOR pathway. Autophagy inhibition resulted in significantly increased cytotoxicity and apoptosis. These findings suggest that air pollution in urban areas may cause nephrotoxicity and autophagy as a protective role in PM-induced cytotoxicity.
