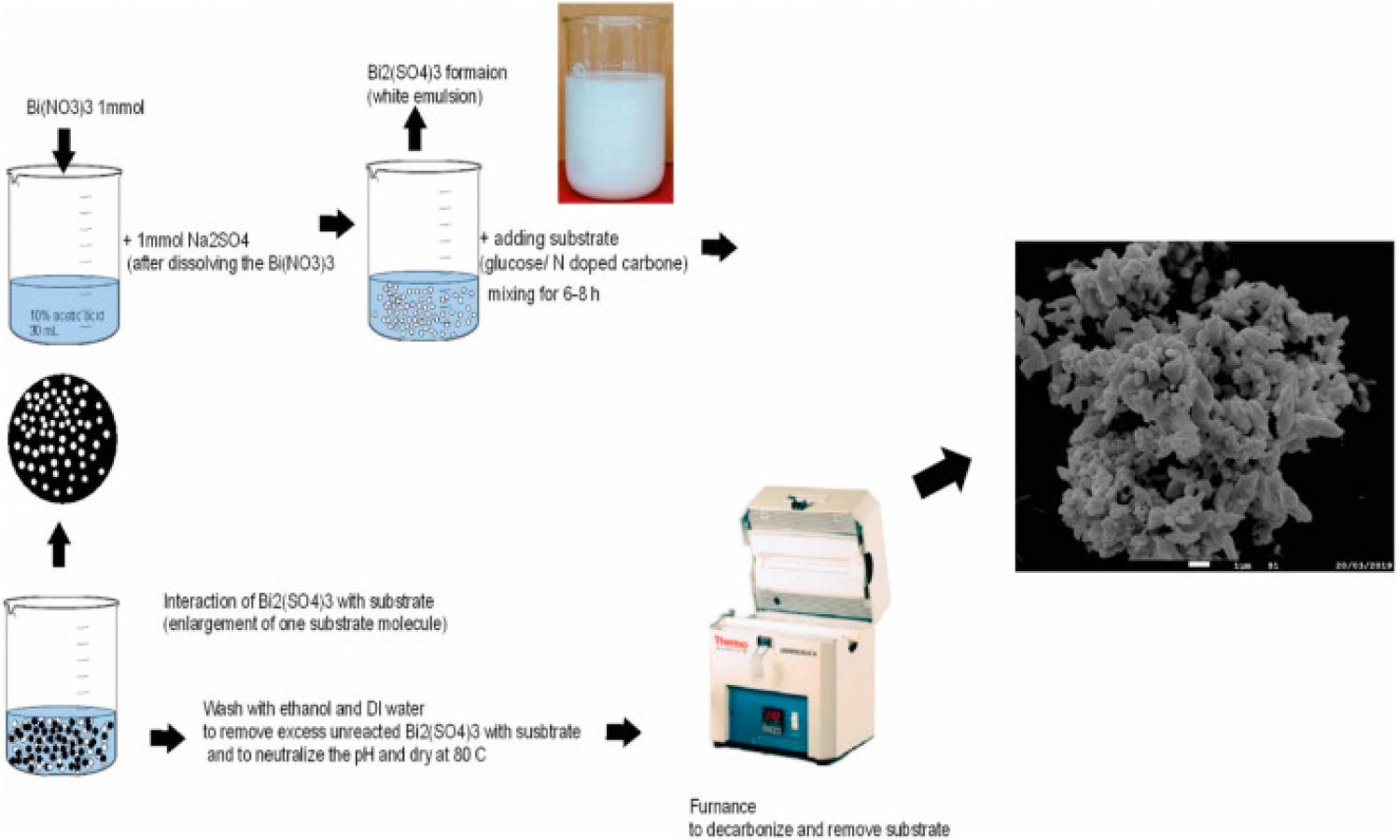
Iodide and bromide ions in surface and ground waters (typically less than 0.05 mg/L for iodide and 0.5 mg/L for bromide) could react with natural organic matters under oxidative disinfection process and produce toxic disinfectant by-products. Therefore, removal of iodides and bromides in water treatment is desirable for improving drinking water quality. We have synthesized a micro-nano Bi2O3-Bi2S3 composite via a carbogenic sphere-supported synthesis strategy. A hydrothermal processing of sucrose resulted in uniform spherical carbogenic microspheres, which served as an excellent substrate for reaction between Bi(NO3)3 and Na2SO4 in emulsion, forming Bi2O3-Bi2(SO4)3 composites, which was then converted into Bi2O3-Bi2S3 after calcination. Using 8 g/L of this material Bi2O3-Bi2S3, 87.69% of iodide at an initial concentration of 0.05 mg/L was removed after 3 h of contact, and 86.38% of bromide at an initial concentration of 0.5 mg/L was removed after 14 h. Such effective removal efficiency was retained when used in artificial groundwater treatment. Overall, this study developed a new method to produce the Bi2O3-Bi2S3 composite in high yield, and the as-synthesized Bi2O3-Bi2S3 composites exhibited high removal efficiencies toward I− and Br− through adsorption mechanism.
