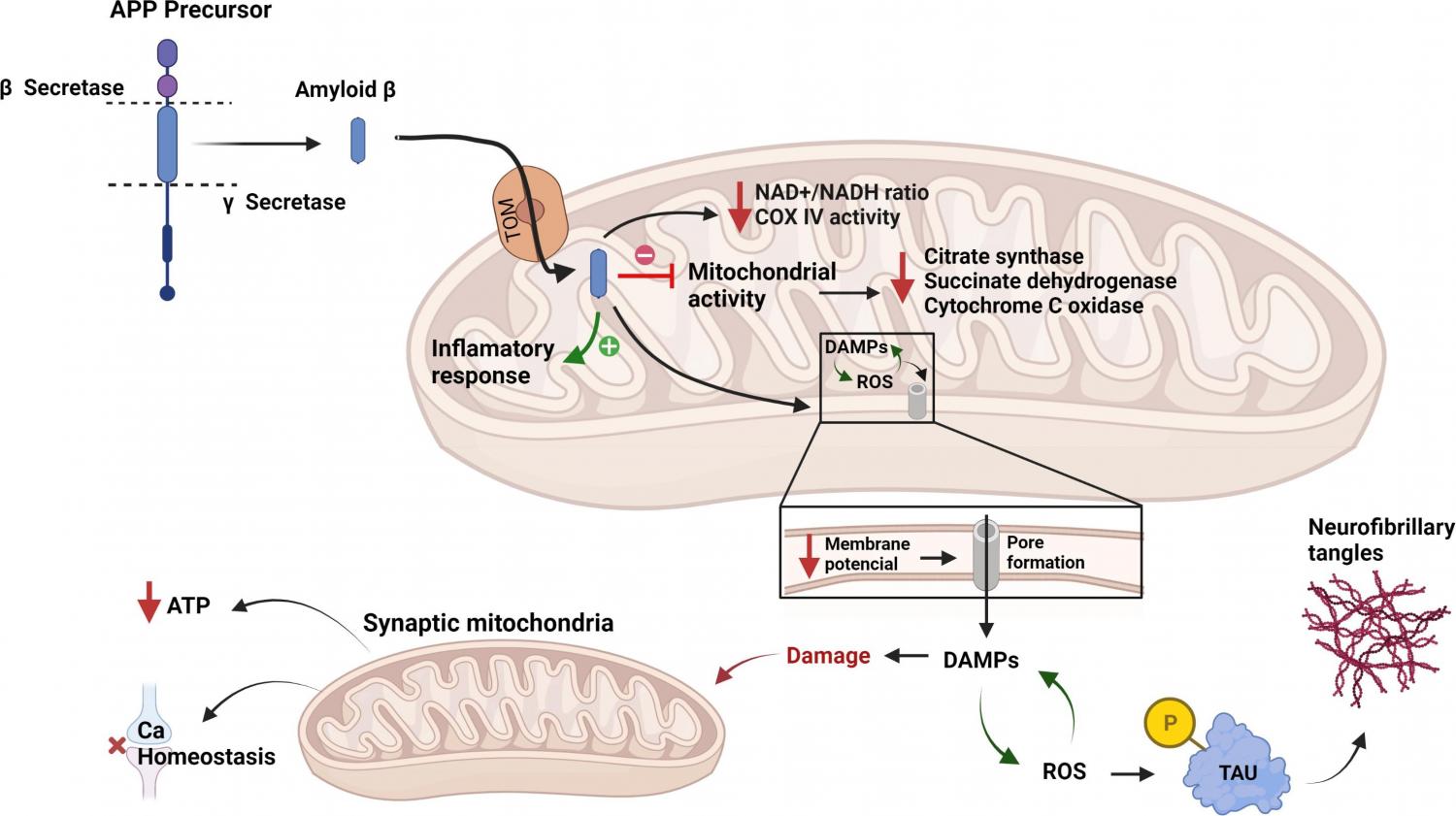
Alzheimer's disease (AD) is a leading neurodegenerative pathology associated with aging worldwide. It is estimated that AD prevalence will increase from 5.8 million people today to 13.8 million by 2050 in the United States alone. AD effects in the brain are well known; however, there is still a lack of knowledge about the cellular mechanisms behind the origin of AD. It is known that AD induces cellular stress affecting the energy metabolism in brain cells. During the pathophysiological advancement of AD, damaged mitochondria enter a vicious cycle, producing reactive oxygen species (ROS), harming mitochondrial DNA and proteins, leading to more ROS and cellular death. Additionally, mitochondria are interconnected with the plaques formed by amyloid-β in AD and have underlying roles in the progression of the disease and severity. For years, the biomedical field struggled to develop new therapeutic options for AD without a significant advancement. However, mitochondria are striking back existing outside cells in a new mechanism of intercellular communication. Extracellular mitochondria are exchanged from healthy to damaged cells to rescue those with a perturbed metabolism in a process that could be applied as a new therapeutic option to repair those brain cells affected by AD. In this review we highlight key aspects of mitochondria's role in CNS’ physiology and neurodegenerative disorders, focusing on AD. We also suggest how mitochondria strikes back as a therapeutic target and as a potential agent to be transplanted to repair neurons affected by AD.