Elsevier, Current Opinion in Chemical Biology, Volume 64, October 2021
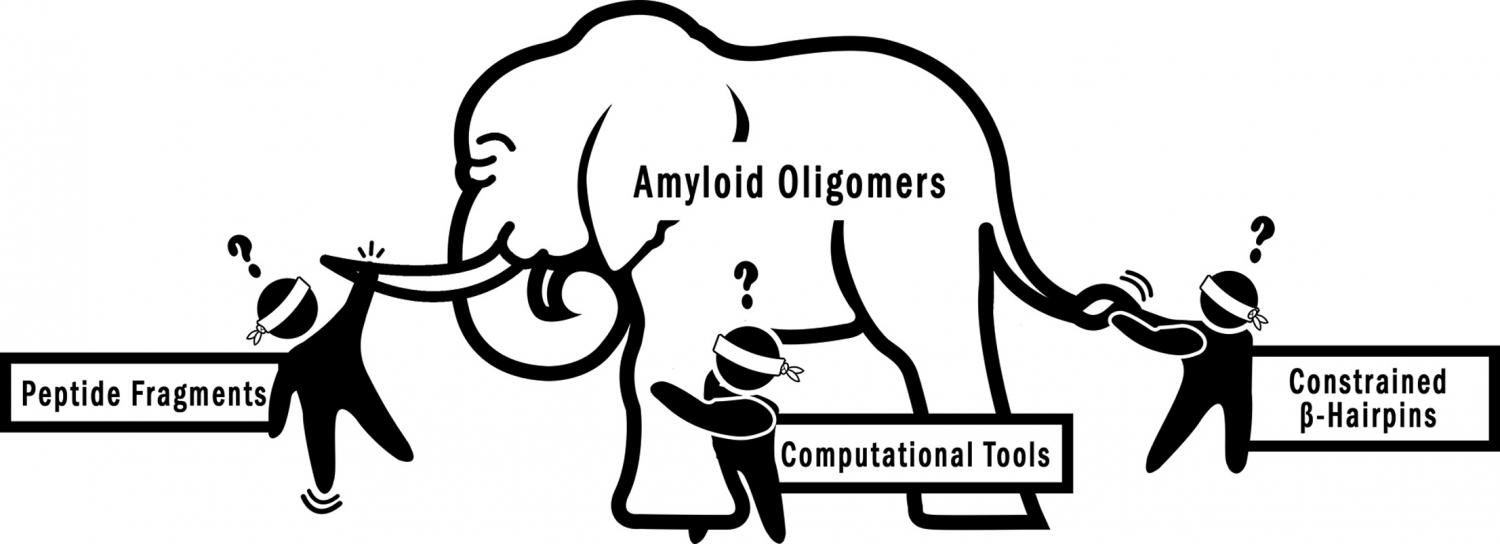
The assembly of amyloidogenic peptides and proteins, such as the β-amyloid peptide, α-synuclein, huntingtin, tau, and islet amyloid polypeptide, into amyloid fibrils and oligomers is directly linked to amyloid diseases, such as Alzheimer's, Parkinson's, and Huntington's diseases, frontotemporal dementias, and type II diabetes. Although amyloid oligomers have emerged as especially important in amyloid diseases, high-resolution structures of the oligomers formed by full-length amyloidogenic peptides and proteins have remained elusive. Investigations of oligomers assembled from fragments or stabilized β-hairpin segments of amyloidogenic peptides and proteins have allowed investigators to illuminate some of the structural, biophysical, and biological properties of amyloid oligomers. Here, we summarize recent advances in the application of these peptide model systems to investigate and understand the structures, biological properties, and biophysical properties of amyloid oligomers.