Elsevier, Neuron, Volume 103, 7 August 2019
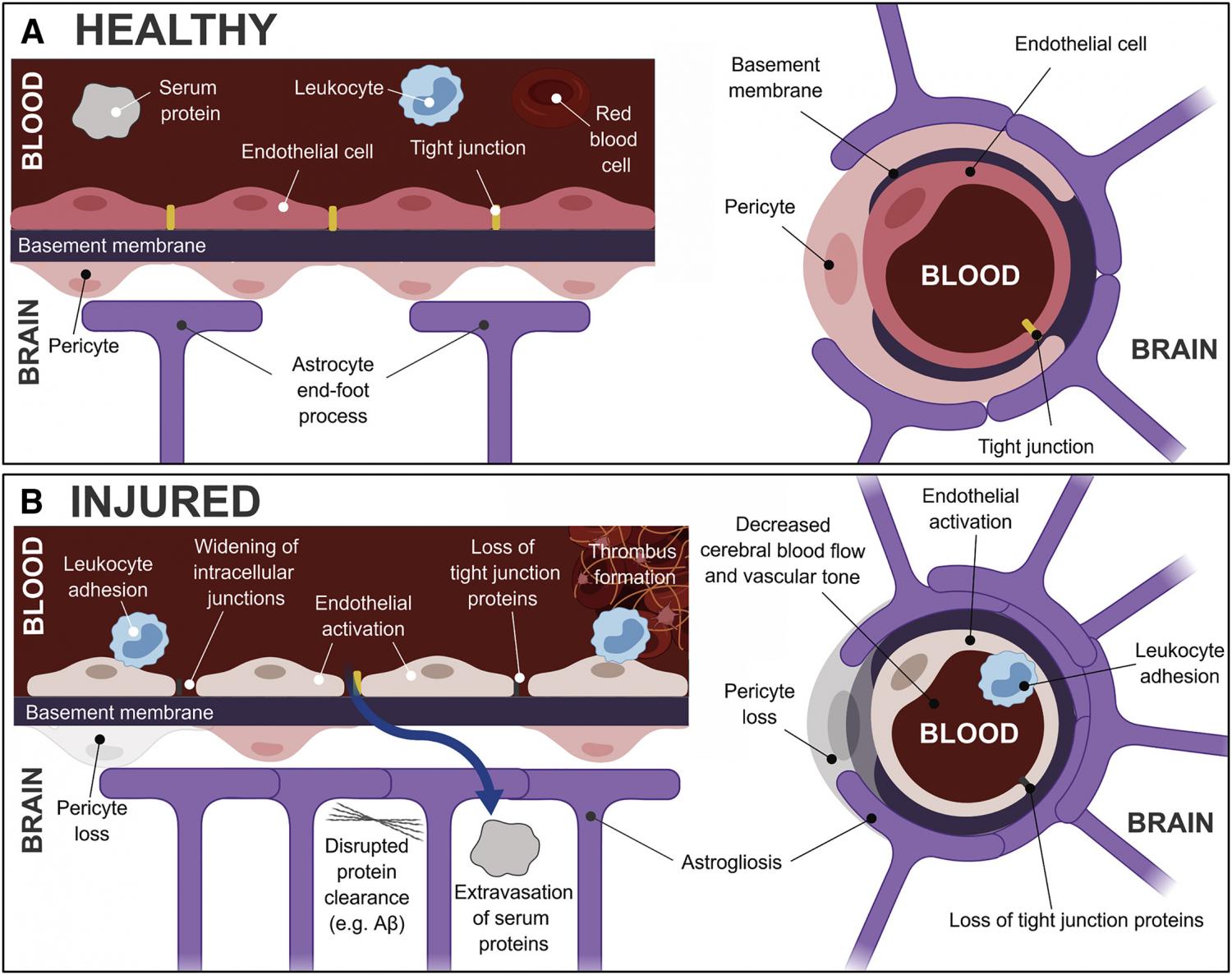
Traumatic brain injury (TBI) is one the most common human afflictions, contributing to long-term disability in survivors. Emerging data indicate that functional improvement or deterioration can occur years after TBI. In this regard, TBI is recognized as risk factor for late-life neurodegenerative disorders. TBI encompasses a heterogeneous disease process in which diverse injury subtypes and multiple molecular mechanisms overlap. To develop precision medicine approaches where specific pathobiological processes are targeted by mechanistically appropriate therapies, techniques to identify and measure these subtypes are needed. Traumatic microvascular injury is a common but relatively understudied TBI endophenotype. In this review, we describe evidence of microvascular dysfunction in human and animal TBI, explore the role of vascular dysfunction in neurodegenerative disease, and discuss potential opportunities for vascular-directed therapies in ameliorating TBI-related neurodegeneration. We discuss the therapeutic potential of vascular-directed therapies in TBI and the use and limitations of preclinical models to explore these therapies. Sandsmark et al. review the evidence of microvascular dysfunction in human and animal TBI, explore the role of vascular dysfunction in neurodegenerative disease, and discuss the potential for vascular-directed therapies in ameliorating TBI-related neurodegeneration.
