Elsevier, Sustainable Materials and Technologies, Volume 22, December 2019
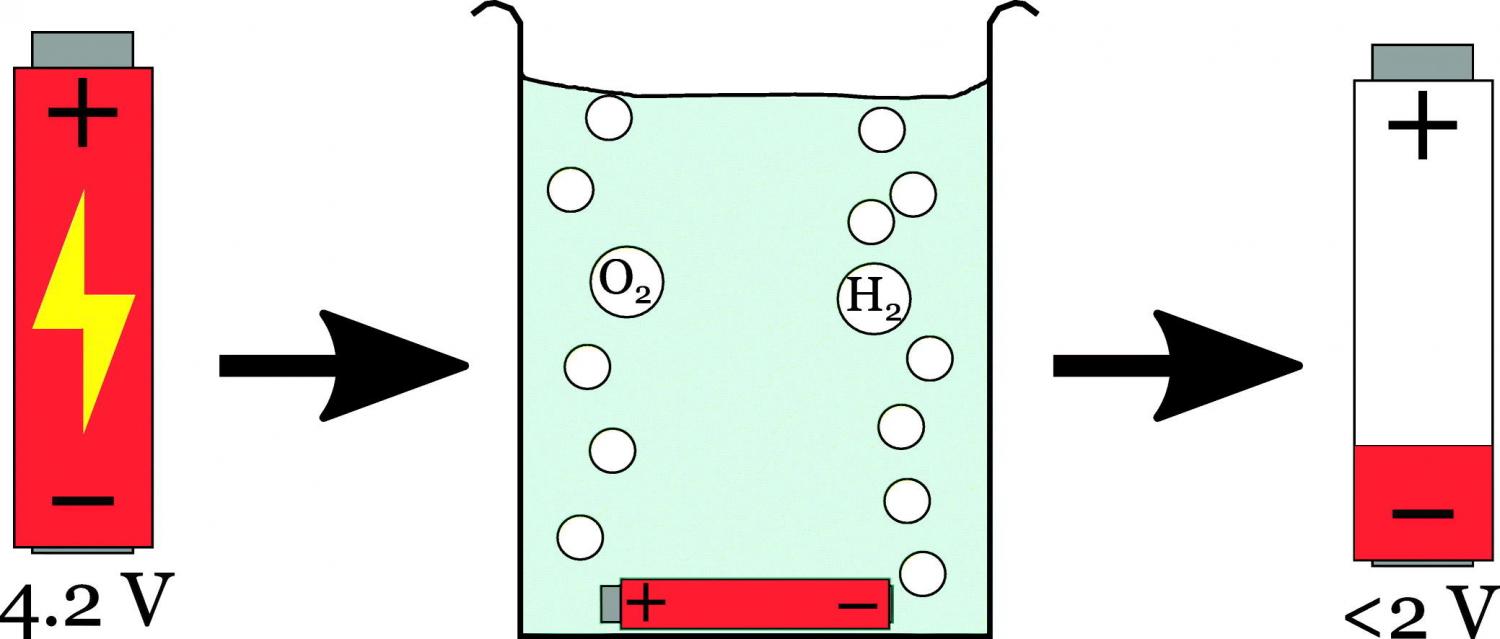
The development of mass-market electric vehicles (EVs) using lithium-ion batteries (LIBs) is helping to propel growth in LIB usage, but end-of-life strategies for LIBs are not well developed. An important aspect of waste LIB processing is the stabilisation of such high energy-density devices, and energy discharge is an obvious way to achieve this. Salt-water electrochemical discharge is often mentioned as the initial step in many LIB recycling studies, but the details of the process itself have not often been mentioned. This study presents systematic discharge characteristics of different saline and basic solutions using identical, fully charged LIB cells. A total of 26 different ionic solutes with sodium (Na+), potassium (K+), and ammonium (NH4+) cations have been tested here using a fixed weight percentage concentration. An evaluation of possible reactions has also been carried out here. The results show good discharge for many of the salts, without significant damaging visual corrosion. The halide salts (Cl−, Br−, and I−) show rapid corrosion of the positive terminal, as does sodium thiosulphate (Na2S2O3), and the solution penetrates the cell can. Mildly acidic solutions do not appear to cause significant damage to the cell can. The most alkaline solutions (NaOH and K3PO4) appear to penetrate the cell without any clear visual damage at the terminals. Depending on what is desired by the discharge (i.e. complete cell destruction and stabilisation or potential re-use or materials recovery), discharge of individual Li-ion cells using aqueous solutions holds clear promise for scaled-up and safe industrial processes.
